Physician Assistant Prescriptive Authority by State (2026 Guide): Controlled Substances, Telemedicine, and Key Drug Rules
Legal & Medical Disclaimer
This content is for informational purposes only and does not constitute legal, medical, or professional advice.
Regulations vary by state and change frequently. The information provided may become outdated. Always verify current regulations with your state board of pharmacy, legal counsel, or regulatory authority before making operational decisions.
RX Agent and its contributors assume no liability for actions taken based on this information. Consult qualified legal and medical professionals for guidance specific to your situation.
Disclaimer: This article is for educational purposes only and does not constitute legal or clinical advice. Always verify your state's requirements.
Understanding Physician Assistant Prescriptive Authority
Physician assistant (PA) prescriptive authority defines what medications PAs can prescribe and under what conditions. Every state grants this authority differently, depending on whether PAs work under supervision, collaboration, transition-to-practice, or independent models.
In practice, can physician assistants prescribe medication? — yes, in all 50 states. However, the degree of autonomy and controlled substance authority varies significantly by jurisdiction.
This 2026 guide synthesizes those differences, focusing on:
- State-level prescriptive models
- Controlled substance schedules (II–V)
- DEA and telemedicine prescribing rules
- Commonly queried drugs like Wegovy, Adderall, and Norco
- The new DEA Special Registration for telehealth providers
The Legal Landscape: Supervision vs. Collaboration
Most states require a defined relationship between PAs and physicians. This can take one of several forms:
| Model | Description |
|---|---|
| Supervised | PA requires direct or indirect oversight from a licensed physician. |
| Collaborative | PA collaborates with a physician, but operational autonomy is higher. |
| Transition-to-Practice | PAs gain independence after completing a specific number of supervised hours. |
| Independent (OTP) | No legal physician tether; scope is defined at the practice level. |
The Optimal Team Practice (OTP) model, pioneered in North Dakota, eliminates the requirement for a formal supervisory agreement. States like Utah, Wyoming, and Montana are moving in this direction by allowing scope determination at the practice level after 8,000–10,000 hours of experience.
Can Physician Assistants prescribe medication without a doctor?
Generally, no — but the tide is shifting.
Most states require PAs to have a supervision or collaboration agreement with a physician to prescribe. However:
- North Dakota grants full independence under Optimal Team Practice.
- Utah and Wyoming determine scope at the practice level, effectively removing state-mandated supervision ratios.
Compared with nurse practitioners (NPs)—many of whom have achieved Full Practice Authority (FPA)—PAs remain more regulated. The PA advocacy community continues to lobby for expanded OTP legislation that allows flexible, team-based scope determination.
For Nurse Practitioner-specific rules and Full Practice Authority states, see our Nurse Practitioner Prescriptive Authority by State guide.
Common Prescribing Questions: Wegovy, Adderall, Norco & More
Patients and providers often ask: What medications can physician assistants prescribe? Here are common examples that illustrate how laws differ:
Can PAs prescribe Wegovy (Semaglutide)?
✅ Yes. Physician assistants can prescribe GLP-1 receptor agonists like Wegovy and Ozempic in all 50 states, provided they have prescriptive authority and, in some states, a collaborating physician agreement.
Can PAs prescribe Adderall?
⚠️ Usually yes, but Adderall is a Schedule II controlled substance, so prescribing rules are stricter. Some states restrict Schedule II prescribing to specific settings (e.g., hospitals or hospice) or impose day-supply limits.
Can PAs prescribe Norco (Hydrocodone/Acetaminophen)?
✅ Yes, though with Schedule II limits. For example:
- Arkansas & Missouri: Limited to hydrocodone combination products only.
- Florida: May prescribe Schedule II opioids but only for a 7-day supply to opioid-naive patients.
Can PAs prescribe peptides or compounded therapies?
⚠️ Only if the peptide is FDA-approved. Compounded peptides face more scrutiny, and PAs must ensure compliance with both federal FDA and state board rules.
Can a Physician Assistant Prescribe Controlled Substances?
Yes—but only within their state’s statutory limits and with the appropriate DEA registration.
Controlled-substance authority follows a two-tier rule:
- State Law: Defines which schedules (II–V) a PA may prescribe and under what conditions.
- Federal Law (DEA): Requires the PA to obtain a DEA registration and follow national prescribing standards.
For example:
- Georgia prohibits PAs from prescribing any Schedule II drug.
- Texas allows it only in hospital or hospice settings.
- North Dakota permits full Schedule II–V prescribing authority under OTP.
The DEA Telemedicine Special Registration: 2026 Update
The DEA’s proposed Special Registration program is transforming telehealth prescribing. It enables PAs and other qualified practitioners to prescribe Schedule II–V medications remotely—without an initial in-person visit—if they meet the following:
- Hold a Special DEA Registration
- Conduct a nationwide PDMP check
- Verify patient identity via real-time audio-video
- Maintain state-specific DEA registration wherever the patient resides
Why It Matters
Under the Ryan Haight Online Pharmacy Act, prescribing controlled substances via telemedicine previously required an in-person evaluation. This was temporarily waived during COVID-19, but those waivers expire at the end of 2025.
The new rule allows compliant PAs to prescribe drugs like Adderall or Testosterone remotely—provided all DEA and state requirements are satisfied.
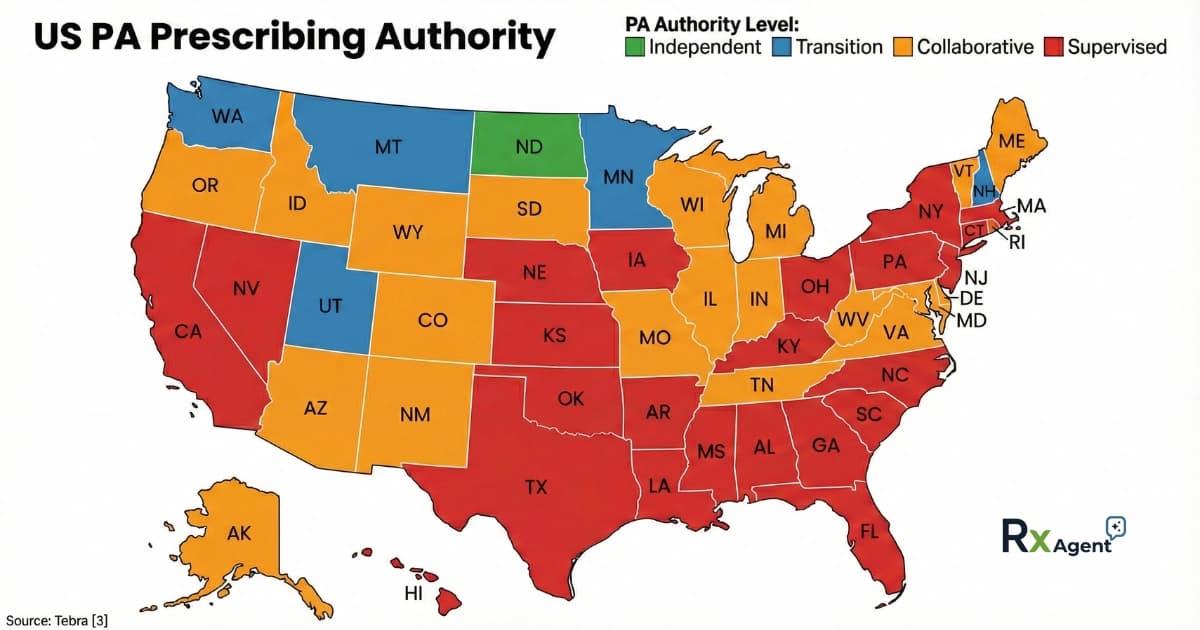
PA Prescribing Authority by State Table (2026)
The following table synthesizes data regarding the practice environment for PAs, specifically focusing on their ability to prescribe controlled substances (CS).
- Independent: No physician relationship mandated by state law
- Transition: Independence allowed after an hours/experience threshold
- Collaborative: Collaborative agreement required
- Supervised: Supervision required
- Sch II: Schedule II Controlled Substances
| State | PA Authority | Key Prescribing Notes |
|---|---|---|
| AL | Supervised | PA: Sch III-V only. Sch II prohibited [3]. |
| AK | Collaborative | PA: Sch II-V requires MD authorization [3]. |
| AZ | Collaborative | PA: Transition to independent after 8k hours [3]. |
| AR | Supervised | Restriction: Sch II limited to hydrocodone products only [3]. |
| CA | Supervised | |
| CO | Collaborative | PA: Sch II limited to 7-day supply for opioid naive [3]. |
| CT | Supervised | PA: Sch II/III req MD approval [3]. |
| DE | Collaborative | PA: Sch II-V authorized [3]. |
| FL | Supervised | PA: Sch II limited to 7-day supply [3]. |
| GA | Supervised | Restriction: PA prohibited from prescribing Sch II [3]. |
| HI | Supervised | PA: Sch II-V under MD supervision [3]. |
| ID | Collaborative | PA: No written or oral prescriptions allowed [3]. |
| IL | Collaborative | |
| IN | Collaborative | PA: Sch II-V requires agreement. No weight loss Sch IIs [3]. |
| IA | Supervised | PA: Sch II depressants req MD approval [3]. |
| KS | Supervised | PA: Sch III-V only [3]. |
| KY | Supervised | |
| LA | Supervised | |
| ME | Collaborative | PA: Independent after 4k hours [3]. |
| MD | Collaborative | |
| MA | Supervised | PA: Sch II requires 96hr MD review [3]. |
| MI | Collaborative | PA: Practice agreement required [3]. |
| MN | Transition | |
| MS | Supervised | |
| MO | Collaborative | Restriction: Sch II limited to hydrocodone. 5-day limit [3]. |
| MT | Transition | PA: Independent after 8k hours [3]. |
| NE | Supervised | |
| NV | Supervised | PA: Sch II-V authorized [3]. |
| NH | Transition | PA: Independent after 8k hours [3]. |
| NJ | Supervised | PA: Sch II-V authorized [3]. |
| NM | Collaborative | PA: Sch II-V authorized [3]. |
| NY | Supervised | |
| NC | Supervised | PA: Sch II/III limited to 30-day supply [3]. |
| ND | Independent | Standout: PA practices independently [3]. |
| OH | Supervised | PA: Sch II restrictions (e.g., terminal illness, MD initiation) [3]. |
| OK | Supervised | Restriction: PA excluded from independent Sch II [3]. |
| OR | Collaborative | PA: Sch II-V authorized [3]. |
| PA | Supervised | PA: Sch II limited to 72hr (initial) or 30-day (ongoing) [3]. |
| RI | Collaborative | PA: Sch II-V authorized [3]. |
| SC | Supervised | Restriction: PA Sch II limited (5-day narcotic/30-day non) [3], [4]. |
| SD | Collaborative | |
| TN | Collaborative | PA: Sch II/III limited to 30-day supply [3]. |
| TX | Supervised | Restriction: Sch II limited to hospital/hospice settings [3]. |
| UT | Transition | PA: Independent after 10k hours [3]. |
| VT | Collaborative | PA: Sch II-V authorized [3]. |
| VA | Collaborative | |
| WA | Transition | PA: Independent after 4k hours [3]. |
| WV | Collaborative | PA: Sch II limited to 3-day supply [3]. |
| WI | Collaborative | |
| WY | Collaborative | PA: Sch II-V authorized [3]. |
PA Prescribing Authority by State Map (2026)
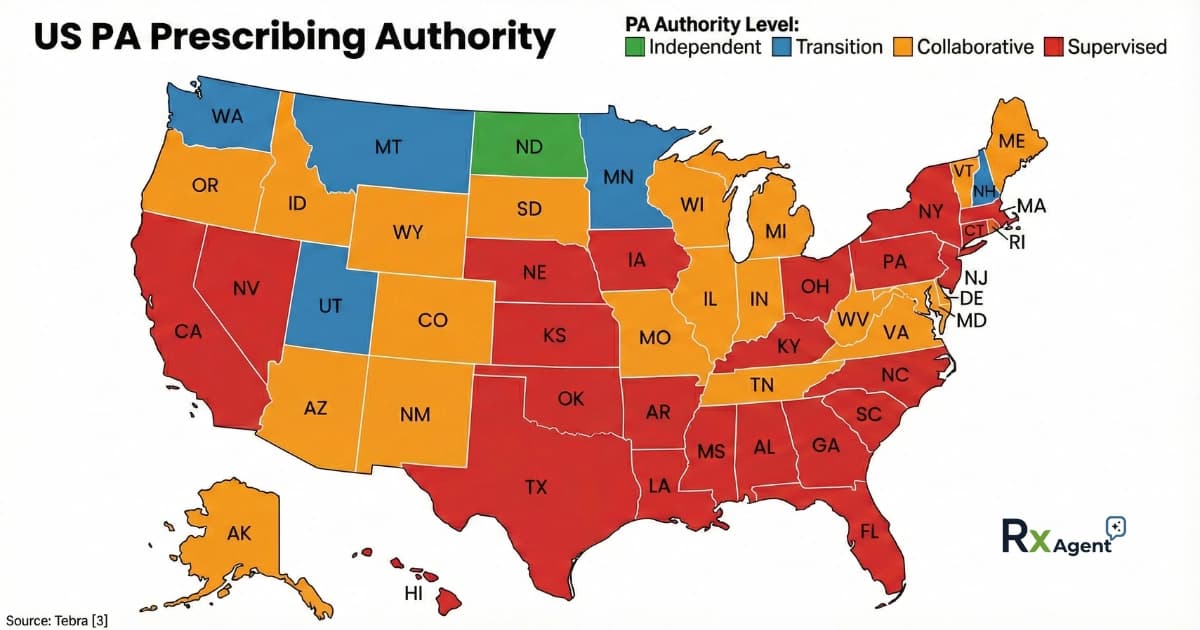
Figure: State-by-state breakdown of Physician Assistant prescribing authority, showing independent, transition, collaborative, and supervised practice environments.
Why Manual Compliance Checks Fail
Healthcare providers working across state lines face a major challenge: the laws change constantly. Manual reference tables, PDFs, and outdated spreadsheets cannot keep up with real-time rule shifts across multiple states and DEA jurisdictions.
Common Provider Risks
- Unknowingly prescribing outside scope (state violation)
- Missing PDMP documentation requirements
- Failing to register with DEA in all states where patients reside
- Inadvertently violating telemedicine prescribing laws
Meet Rx Agent — Your AI Compliance Tool
Rx Agent is the best telehealth compliance software designed for prescribers and telemedicine organizations.
It acts as your automated compliance officer, helping you:
- Instantly verify state-specific prescriptive authority
- Cross-reference DEA and telemedicine laws
- Get real-time updates when regulations change
- Eliminate manual cross-checking and compliance errors
👉 Sign up for Early Access and simplify your multi-state prescribing compliance today.
Conclusion: The Future of PA Prescribing Authority
The landscape of physician assistant prescriptive authority by state is rapidly evolving toward greater autonomy.
As Optimal Team Practice expands and DEA telemedicine frameworks mature, PAs are gaining more flexibility—paired with higher compliance accountability.
The key takeaway for PAs and telemedicine providers:
✅ Stay informed, stay compliant, and use automated tools, like Rx Agent, to navigate state prescribing laws.
References
1. https://www.aanp.org/advocacy/advocacy-resource/policy-briefs/issues-full-practice-brief
2. https://nurse.org/education/np-full-practice-authority/
3. https://www.tebra.com/theintake/checklists-and-guides/legal-and-compliance/nurse-practitioner-laws-by-state
4. https://www.scstatehouse.gov/billsearch.php?billnumbers=3580
5. https://nacns.org/wp-content/uploads/2020/08/PractPrescAuthority7.31.2020.pdf
6. https://www.dlapiper.com/insights/publications/2025/01/dea-and-hhs-publish-rules-for-telemedicine-prescriptions
7. https://www.govinfo.gov/content/pkg/FR-2025-01-17/pdf/2025-01099.pdf
Frequently Asked Questions
About the Author
Dr. Zade Shammout, PharmD writes about prescription medications, pharmacy laws, and healthcare compliance for prescribers and pharmacists.